KIT, NRAS and BRAF mutations in melanoma
Keywords:
Melanoma, mutations, genetic alterations, KIT, NRAS, BRAF.Abstract
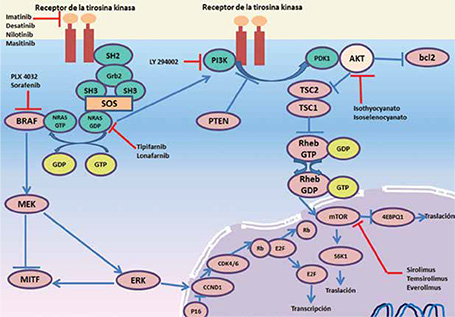
Melanoma is one of the least common skin cancers, but given its ability to metastasize corresponds to the leading cause of death from skin cancer. There are five major clinical subtypes, of which the most common in Colombia is acral lentiginous melanoma. In the last decades altered intracellular signaling pathways in the pathogenesis of melanoma have been detected, with gen mutations that primarily affect KIT, NRAS and BRAF more often, all involved in the activation of the MAPK signaling pathway genes. Thanks to these discoveries that selectively inhibit the transcription of these genes drugs have been developed and mutants in phase II clinical trials have demonstrated a decrease in tumor size and increased survival of patients with metastatic melanoma.
Author Biographies
Luz de María Díaz-Granados
Médica, residente de tercer año de Dermatología, Sección de Dermatología, Universidad de Antioquia: Grupo de Investigación Dermatológica, GRID, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Margarita María Velásquez
Médica dermatóloga y doctora en Ciencias Básicas Biomédicas; profesora, Sección de Dermatología, Centro de Investigaciones Dermatológicas –CIDERM, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia.
References
2. Acosta Á, Fierro E, Velásquez V, Rueda X. Melanoma: patogénesis, clínica e histopatología. Rev Asoc Col Dermatol. 2009;17:87-108.
3. Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-74.
4. Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3-9.
5. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
6. Tuong W, Cheng LS, Armstrong AW. Melanoma: Epidemiology, diagnosis, treatment, and outcomes. Dermatol Clin. 2012;30:113-24.
7. Geller AC, Annas GD. Epidemiology of melanoma and nonmelanoma skin cancer. Semin Oncol Nurs. 2003;19:2-11.
8. Globocan. Cancer incidence and mortality worldwide in 2008 [Internet] France [updated October, 2012. Fecha de consulta: octubre 3 de 2015. Disponible en: http:// globocan.iarc.fr/factsheet.asp.
9. Volkovova K, Bilanicova D, Bartonova A, Letasiova S, Dusinska M. Associations between environmental factors and incidence of cutaneous melanoma. Review. Environ Health. 2012;11(Suppl.1):S12.
10. Erdmann F, Lortet-Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953-2008--are recent generations at higher or lower risk? Int J Cancer. 2013;132:385-400.
11. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: A systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69-82.
12. Green A, Autier P, Boniol M, Boyle P, Dor J-F, Gandini S, et al. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120:1116-22.
13. Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005;23:2669-75.
14. Hemminki K, Zhang H, Czene K. Familial and attributable risks in cutaneous melanoma: Effects of proband and age. J Invest Dermatol. 2003;120:217-23.
15. Ferrone CR, Ben Porat L, Panageas KS, Berwick M, Halpern AC, Patel A, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294:1647-54.
16. MacKie RM, Hauschild A, Eggermont AM. Epidemiology of invasive cutaneous melanoma. Ann Oncol. 2009;20(Suppl.6):vi1-7.
17. Olsen CM, Carroll HJ, Whiteman DC. Estimating the attributable fraction for cancer: A meta-analysis of nevi and melanoma. Cancer Prev Res (Phila). 2010;3:233-45.
18. Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843-50.
19. Kraemer KH, DiGiovanna JJ. Xeroderma pigmentosum. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. Gene Reviews. Seattle (WA): University of Washington, Seattle; 1993.
20. Kvaskoff M, Mesrine S, Fournier A, Boutron-Ruault MC, Clavel-Chapelon F. Personal history of endometriosis and risk of cutaneous melanoma in a large prospective cohort of French women. Arch Intern Med. 2007;167:2061-5.
21. Olsen JH, Friis S, Frederiksen K, McLaughlin JK, Mellemkjaer L, Moller H. Atypical cancer pattern in patients with Parkinson’s disease. Br J Cancer. 2005;92:201-5.
22. Restrepo C, Velásquez M. Mecanismos de patogénesis del melanoma maligno. Rev Asoc Colomb Dermatol. 2012;20:161-72.
23. Zwald FO, Christenson LJ, Billingsley EM, Zeitouni NC, Ratner D, Bordeaux J, et al. Melanoma in solid organ transplant recipients. Am J Transplant. 2010;10:1297-304.
24. Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370-3.
25. Erickson CA, Reedy MV. Neural crest development: The interplay between morphogenesis and cell differentiation. Curr Top Dev Biol. 1998;40:177-209.
26. Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095-104.
27. Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76-83.
28. Bandarchi B, Jabbari CA, Vedadi A, Navab R. Molecular biology of normal melanocytes and melanoma cells. J Clin Pathol. 2013;66:644-8.
29. Uong A, Zon LI. Melanocytes in development and cancer. J Cell Physiol. 2010;222:38-41.
30. Yasumoto K, Yokoyama K, Shibata K, Tomita Y, Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994;14:8058-70.
31. Wehrle-Haller B, Weston JA. Soluble and cell-bound forms of steel factor activity play distinct roles in melanocyte precursor dispersal and survival on the lateral neural crest migration pathway. Development. 1995;121:731-42.
32. Giebel LB, Spritz RA. Mutation of the KIT (mast/stem cell growth factor receptor) protooncogene in human piebaldism. Proc Natl Acad Sci U S A. 1991;88:8696-9.
33. Kilsby AJ, Cruwys M, Kukendrajah C, Russell-Eggitt I, Raglan E, Rajput K, et al. Homozygosity for piebaldism with a proven KIT mutation resulting in depigmentation of the skin and hair, deafness, developmental delay and autism spectrum disorder. Clin Dysmorphol. 2013;22:64-7.
34. van den Hurk K, Niessen HE, Veeck J, van den Oord JJ, van Steensel MA, Zur Hausen A, et al. Genetics and epigenetics of cutaneous malignant melanoma: A concert out of tune. Biochim Biophys Acta. 2012;1826:89-102.
35. Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, Louis G, et al. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J Biol Chem. 2003;278:9790-5.
36. Da Forno PD, Saldanha GS. Molecular aspects of melanoma. Clin Lab Med. 2011;31:331-43.
37. Held L, Eigentler TK, Meier F, Held M, Rocken M, Garbe C, et al. Oncogenetics of melanoma: Basis for molecular diagnostics and therapy. J Dtsch Dermatol Ges. 2011;9:510-6.
38. Fernández M, Della-Giovanna P. Genodermatosis relacionadas con la vía RAS/MAPK. Arch Argent Dermatol. 2011;61:185-90.
39. Solus JF, Kraft S. Ras, Raf, and MAP kinase in melanoma. Adv Anat Pathol. 2013;20:217-26.
40. Kong Y, Kumar SM, Xu X. Molecular pathogenesis of sporadic melanoma and melanoma-initiating cells. Arch Pathol Lab Med. 2010;134:1740-9.
41. Swick JM, Maize JC, Sr. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-54.
42. Mehnert JM, Kluger HM. Driver mutations in melanoma: Lessons learned from bench-to-bedside studies. Curr Oncol Rep. 2012;14:449-57.
43. Flaherty KT, Fisher DE. New strategies in metastatic melanoma: Oncogene-defined taxonomy leads to therapeutic advances. Clin Cancer Res. 2011;17:4922-8.
44. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340-6.
45. Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14:6821-8.
46. Rivera RS, Nagatsuka H, Gunduz M, Cengiz B, Gunduz E, Siar CH, et al. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452:27-32.
47. Kudchadkar RR, Smalley KS, Glass LF, Trimble JS, Sondak VK. Targeted therapy in melanoma. Clin Dermatol. 2013;31:200-8.
48. Malumbres M, Barbacid M. RAS oncogenes: The first 30 years. Nat Rev Cancer. 2003;3:459-65.
49. Takata M, Saida T. Genetic alterations in melanocytic tumors. J Dermatol Sci. 2006;43:1-10.
50. Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 2006;66:9483-91.
51. Padua RA, Barrass N, Currie GA. A novel transforming gene in a human malignant melanoma cell line. Nature. 1984;311:671-3.
52. Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: Mutations occur early and persist throughout tumor progression. Clin Cancer Res. 2002;8:3468-74.
53. Sheen YS, Liao YH, Liau JY, Lin MH, Hsieh YC, Jee SH, et al. Prevalence of BRAF and NRAS mutations in cutaneous melanoma patients in Taiwan. J Formos Med Assoc. 2015;¿volume?:¿pages?
54. Johnson DB, Sosman JA. Update on the targeted therapy of melanoma. Curr Treat Options Oncol. 2013;14:280-92.
55. Woodman SE, Lazar AJ, Aldape KD, Davies MA. New strategies in melanoma: Molecular testing in advanced disease. Clin Cancer Res. 2012;18:1195-200.
56. Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-46.
57. Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720-4.
58. Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412-21.
59. Handolias D, Hamilton AL, Salemi R, Tan A, Moodie K, Kerr L, et al. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer. 2010;102:1219-23.
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






