Biopsia de mucosa nasal: descripción de técnicas y comparación de los resultados con gubia versus otros instrumentos en un hospital de referencia
DOI:
https://doi.org/10.29176/2590843X.1550Keywords:
Biopsy, Diagnostic, Nasal mucosa, Procedures, TechniquesAbstract
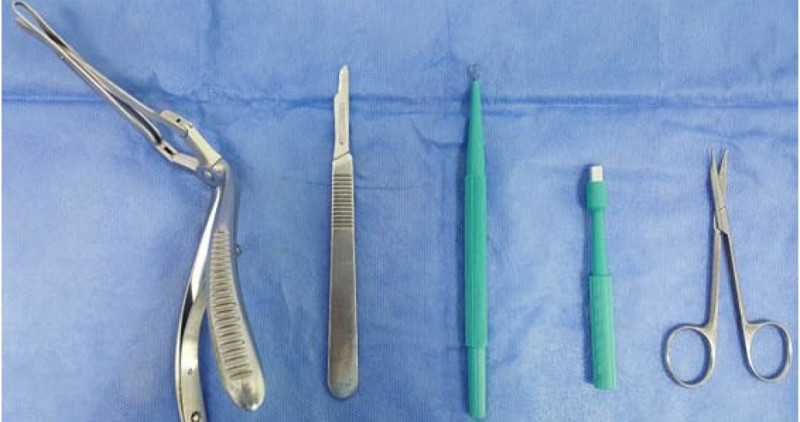
Objectives: For the nasal mucosa biopsy, various instruments are used, and the histological result may vary. The purpose of this study is to describe and determine the frequency of satisfactory histological results of biopsy taken with gouge versus other instruments in patients with nasal mucosal lesions.
Materials and methods: Cross-sectional study; included clinical records of patients who underwent nasal mucosa biopsy between 2005 to 2016 in a dermatological hospital in Bogotá, Colombia. Extra-institutional biopsies were excluded. Demographic, clinical and paraclinical characteristics were analyzed. The satisfactory result was defined as obtaining a sufficient sample without the presence of artifices that can prevent the pathologist from confirming the diagnosis. Additionally, diameter and depth of samples were reported.
Results: 98 records were included. In 70.4% of the cases biopsy was taken with gouge. The diameter of the sample with gouge was slightly larger (3.2 mm vs 2.8 mm [p=0.16]). The frequency of satisfactory results was similar in those who used gouge (94.2%) or other instruments (93.1%). However, the biopsies with satisfactory results were of greater diameter and depth.
Discussion and conclusions: No differences were observed in the frequency of satisfactory results according to the type of instruments, probably the quality of the sample is operator dependent. To increase the probability of a satisfactory result, it is suggested to have a sample with a diameter greater than three mm and at least two mm deep.
Author Biographies
Diana Patricia Crizón-Díaz
Posgrado de Dermatología, Fundación Universitaria Sanitas, Bogotá, Colombia. Hospital Universitario Centro Dermatológico Federico Lleras Acosta, Bogotá, Colombia
Victoria Eugenia Franco
Posgrado de Dermatología, Fundación Universitaria Sanitas, Bogotá, Colombia. Hospital Universitario Centro Dermatológico Federico Lleras Acosta, Bogotá, Colombia
Claudia Carolina Colmenares
Unidad de investigación, Fundación Universitaria Sanitas, Bogotá, Colombia.
References
2. Gul HC, Tosun F, Karakas A, Koru O, Onguru O, Mert G, et al. A case of mucosal leishmaniasis: Mimicking intranasal tumor with perforation of septum. J Microbiol Immunol Infect. 2016;49(4):604-7. doi: 10.1016/j.jmii.2013.11.007
3. Prior AJ, Calderon MA, Lavelle RJ, Davies RJ. Nasal biopsy: indications, techniques and complications. Respir Med. 1995;89(3):161-9. doi: 10.1016/0954-6111(95)90242-2
4. Rodriguez G, Pinto R. La lepra. Imágenes y conceptos. Medellín: Editorial Universidad de Antioquia; 2007.
5. Truman R, Krahenbuhl J. Viable M. leprae as a research reagent. Int J Lepr Other Mycobact Dis. 2001;69(1):1-12.
6. Milanes R, Caro C, Vélez C, Marrugo O. Diagnóstico diferencial de la rinosporidiosis: a propósito de un caso. Iatreia. 2012;25(3):272-6.
7. Jaimes A, Muvdi S, Alvarado Z, Rodríguez G. Perforation of the nasal septum as the first sign of histoplasmosis associated with AIDS and review of published literature. Mycopathologia. 2013;176(1-2):145-50. doi: 10.1007/s11046-013-9662-z
8. Reyes HD, Marquez RE, Fuste JC, Ramos VJ. Granuloma letal de la línea media como diagnóstico diferencial de la vasculitis de Wegener. Presentación de una paciente. Acta Med Centro. 2015;9(2):34-40.
9. Mallya V, Singh A, Pahwa M. Lethal midline granuloma. Indian Dermatol Online J. 2013;4(1):37-9. doi: 10.4103/2229-5178.105469
10. Fuller J. Selección del instrumental quirúrgico. En: Fuller J, Ness E (editores). Instrumentación quirúrgica: teoría, técnicas y procedimientos. 4.ª edición. México: Editorial Médica Panamericana; 2007. p. 457-9.
11. Zuber TJ. Fusiform excision. Am Fam Physician. 2003;67(7):1539-44, 1547–8, 1550.
12. Goldberg LH, Alam M. Elliptical excisions: variations and the eccentric parallelogram. Arch Dermatol. 2004;140(2):176-80. doi: 10.1001/archderm.140.2.176
13. Pickett H. Shave and punch biopsy for skin lesions. Am Fam Physician. 2011;84(9):995-1002.
14. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20(3):569-78, ix. doi: 10.1016/s0733-8635(02)00022-0
15. StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.
16. Segal N, Osyntsov L, Olchowski J, Kordeluk S, Plakht Y. Nose biopsy: a comparison between two sampling techniques. Eur Arch Otorhinolaryngol. 2016;273(6):1445-8. doi: 10.1007/s00405-015-3754-y
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section

| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






