Hypertricosis as a dermatological adverse effect associated to the use of panitumumab
DOI:
https://doi.org/10.29176/2590843X.61Abstract
Panitumumab is a type of Epidermal Growth Factor Receptor Inhibitor (EGFRI) that belongs to the new antineoplastic therapeutic strategies associated with dermatological adverse effects. Panitumumab has been used for the treatment
of metastatic colon cancer.
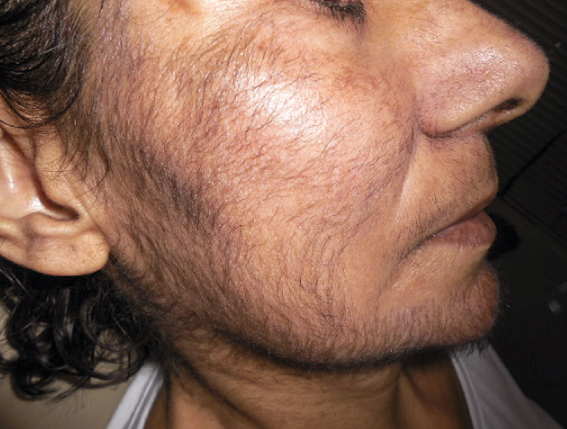
We report the case of hypertrichosis secondary to panitumumab and we discuss its physiopathology.
Author Biographies
Sandra Patricia Herrera, Clínica Foscal Internacional FOSUNAB
Médica dermatóloga, Fundación Oftalmológica de Santander-Clínica Carlos Ardila Lülle (FOSCAL), Clínica Foscal Internacional FOSUNAB,Insuasty Oncología, Bucaramanga, Colombia
Natalia Andrea Rueda, Clínica Foscal Internacional FOSUNAB
Médica general, Fundación Oftalmológica de Santander-Clínica Carlos Ardila Lülle (FOSCAL), Clínica Foscal Internacional FOSUNAB, Insuasty Oncología, Bucaramanga, Colombia
Jennifer Paola Rueda, Clínica Foscal Internacional FOSUNAB
Médica general, Fundación Oftalmológica de Santander-Clínica Carlos Ardila Lülle (FOSCAL), Clínica Foscal Internacional FOSUNAB, Insuasty Oncología, Bucaramanga, Colombia
Jesús Insuasty3, Clínica Foscal Internacional FOSUNAB
Médico internista y oncólogo clínico, Fundación Oftalmológica de Santander-Clínica Carlos Ardila Lülle (FOSCAL), Clínica Foscal Internacional FOSUNAB, Insuasty Oncología, Bucaramanga, Colombia
Ricardo Flaminio Rojas, Clínica Foscal Internacional FOSUNAB
Médico dermatólogo, Fundación Oftalmológica de Santander-Clínica Carlos Ardila Lülle (FOSCAL), Clínica Foscal Internacional FOSUNAB, Insuasty Oncología, Bucaramanga, Colombia
References
Cohen PR, Escudier SM, Kurzrock R. Cetuximabassociated elongation of the eyelashes. Am J Clin Dermatol. 2011;12:63-7. https://doi.org/10.2165/11531920-000000000-00000
Françoso A, Simioni PU. Immunotherapy for the treatment of colorectal tumors : Focus on approved and in-clinical-trial monoclonal antibodies. Drug Des Devel Ther. 2017;11:177-84. https://doi.org/10.2147/DDDT.S119036
Lacouture ME, Anadkat M, Jatoi A, Garawin T, Bohac C, Mitchell E. Dermatologic toxicity occurring during anti-EGFR monoclonal inhibitor therapy in patients with metastatic colorectal cancer: A systematic review. Clin Colorectal Cancer. 2018;17:85-96. https://doi.org/10.1016/j.clcc.2017.12.004
Wu PA, Balagula Y, Lacouture ME, Anadkat MJ. Prophylaxis and treatment of dermatologic adverse events from epidermal growth factor receptor inhibitors. Curr Opin Oncol. 2011;23:343-51. https://doi.org/10.1097/CCO.0b013e3283474063
Alexandrescu DT, Kauffman CL, Dasanu CA. The cutaneous epidermal growth factor network: Can it be translated clinically to stimulate hair growth? Dermatol Online J. 2009;15:1-6.
Glas J, Török HP, Folwaczny C, Schneider A, Stolte M, Brünnler G, et al. Abnormal hair growth in a patient with head and neck cancer treated with the anti-epidermal growth factor receptor monoclonal antibody cetuximab. J Clin Oncol. 2005;23:5272-3. https://doi.org/10.1200/JCO.2005.01.5578
Cignola S, Gonella S, Alessandra B, Palese A. Monoclonal antibody-induced papulopustular rash: Clinical course, communication to health-care professionals and reactive measures as reported by patients. Eur J Oncol Nurs.2016;20:133-9. https://doi.org/10.1016/j.ejon.2015.07.003
Berner D, Schlegel C, Metzler G, Ro M. Facial hypertrichosis induced by cetuximab, an antiEGFR monoclonal antibody. JAMA Dermatol. 2006;142:1656-7.
Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-19. https://doi.org/10.1007/s11523-009-0114-0
Baykal C, Babuna kobaner G. Hypertrichosis of the pinnae in a patient using panitumumab. J Eur Acad Dermatol Venereol. 2018;32:e277-8. https://doi.org/10.1111/jdv.14815
Wendelin DS, Pope DN, Mallory SB. Hypertrichosis. J Am Acad Dermatol. 2003;48(Suppl.2):161-79. https://doi.org/10.1067/mjd.2003.100
Imbernón-Moya A, Podlipnik S, Burgos F, VargasLaguna E, Aguilar-Martínez A, Fernández-Cogolludo E, et al. Acquired localized hypertrichosis induced by rivastigmine. Case Rep Dermatol Med. 2016;2016:1-3. https://doi.org/10.1155/2016/7296572
Wollenberg A, Kroth J, Hauschild A, Dirschka T. Hautreaktionen unter EGFR-Inhibitoren - Klinik und Management. DMW - Dtsch Medizinische Wochenschrift. 2010;135:149-54. https://doi.org/10.1055/s-0029-1244831
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






