Biología de la cicatrización
Keywords:
cicatrización, factores de crecimiento, citoquinas, colágenoAbstract
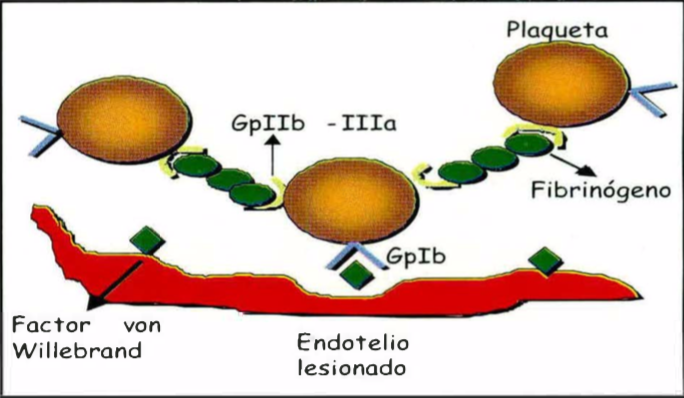
Las heridas se clasifican, según el tiempo de evolución en agudas y crónicas, y según su extensión en heridas de espesor parcial y total. El proceso de cicatrización se divide en cuatro fases: l. Hemostasia, donde las plaquetas, y la cascada de coagulación, además de cumplir funciones hemostáticas, aportan una matriz y una serie de moléculas proinflamatorias necesarias para iniciar la reparación. 11. Inflamación, donde polimorfonucleares, monocitos y linfocitos se encargan de debridar y liberar factores de crecimiento y citoquinas para estimular los procesos siguientes. 111. Proliferación, se reemplazan todos los tejidos perdidos en la herida por medio del proceso de re-epitelización, fibroplasia y angiogénesis, y IV. Remodelación, donde se remodela la cicatriz y recupera la fuerza tensil. Para que todos estos procesos puedan ser llevados a cabo exitosamente, se requiere de factores de crecimiento y citoquinas producidos por una gran variedad de células, cada uno con receptores específicos y funciones determinadas que regulan la proliferación, migración y diferenciación celular.
Author Biography
Adriana Cruz, Universidad del Valle
Residente JI Dermatología, Universidad del Valle, Cali.
References
Falabella AF, Valencia IC. Aspectos básicos en la cicatrización de las heridas. Med Cutan lber Lat Am 2000; 28:144-161.
Mertz PM. Experimental animal wound models. Wounds 2001;13:9-23.
Kirsner R, Eaglstein W. The wound healing process. Dermatol Clin 1993; 11:629-640.
https://doi.org/10.1016/S0733-8635(18)30216-X
Falabella AF, Falanga V. Wound Healing. Biology of the Skin 2001;19:281-297.
Clark RA. Mechanisms of cutaneous wound repair. En: Fitzpatrick T, Eisen A, Wolff K, Freedberg 1, Austen K. Dermatology in General Medicine, New York, McGraw-Hill 1999:326-341.
Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: Cooperative processing of extracellular information. Curr Opin Cell Biol 1992; 4:772-781.
https://doi.org/10.1016/0955-0674(92)90100-Q
Huhtala P, Humphries MJ, McCarthy JB, et al. Cooperative signaling by a5P1 and a4P1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol 1995;129:867-879.
https://doi.org/10.1083/jcb.129.3.867
Tremble P, Damsky CH, Werb Z. Components of the nuclear signaling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol 1995;129:1707-1720.
https://doi.org/10.1083/jcb.129.6.1707
Xu J, Clark RA. Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol 1996; 132:239-249.
https://doi.org/10.1083/jcb.132.1.239
O. Cotran RS, Kumar V, Collins T. Tissue repair: cellular growth, fibrosis and wound healing. En: Robbins. Pathologic Basis of Disease. WB Saunders, Filadelfia 1999; 89:111.
Olman MA, Mackman N, Gladson CL, et al. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin lnvest 1995; 96:1621-1630.
https://doi.org/10.1172/JCI118201
Eltzman DT, McCoy RD, Zheng X, et al. Bleomyc-ininduced fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor 1-gene. J Clin lnvest 1996; 97:232-237.
https://doi.org/10.1172/JCI118396
Gresham HD, Goodwin JL, Allen PM, et al. A novel member of the integrin receptor family mediates Arg-Gly-Asp stimulated neutrophil phagocytosis. J Cell Biol 1989; 108:1935-1943.
https://doi.org/10.1083/jcb.108.5.1935
Simpson DM, Ross R. The neutrophilic leukocyte in wound repair. A study with antineutrophil serum. J Clin lnvest 1972; 51 :2009-2023.
https://doi.org/10.1172/JCI107007
Clark AA. Wound repair, overview and general considerations. En: The Molecular and Cellular Biology of Wound Aepair. AA Clark, New York, Plenum, 1996:3-50.
https://doi.org/10.1007/978-1-4899-0185-9_1
Weller K, Knop J, Maurer M. Mast cells promete wound healing by initiating inflammation. J Clin lnvest 2001; 117:764-820.
Hebda PA, CollinsMA, TharpMD.Mast cell and myofibroblast in wound healing. Dermatol Clin 1993; 11:685-696.
https://doi.org/10.1016/S0733-8635(18)30221-3
Gabbiani G, Chapponnier C, Huttner l. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J Cell Bici 1978; 76:561-568.
https://doi.org/10.1083/jcb.76.3.561
Freedberg IM, Tumic-Canic M, Komine M, et al.Keratins and the keratinocyte activation cycle. J Clin lnvest 2001; 116:633-640.
https://doi.org/10.1046/j.1523-1747.2001.01327.x
Woodley DT, Bachmann PM, O'Keefe EJ. Laminin inhibits human keratinocyte migration. J Cell Physiol 1988;136:140-146.
https://doi.org/10.1002/jcp.1041360118
Woodley DT, Chen JO, Kim JP, et al. Re-epithelialization, human keratinocyte locomotion. Oermatol Clin 1993; 11:641-646.
https://doi.org/10.1016/S0733-8635(18)30217-1
Larjava H, Salo T, Haapassaimi K, et al. Expression of integrins and basement membrane components by wound keratinocytes. J Clin lnvest 1993; 92:1425-1435.
https://doi.org/10.1172/JCI116719
Clark RA. Biology of dermal wound repair. Dermatol Clin 1993; 11:647-666.
https://doi.org/10.1016/S0733-8635(18)30218-3
Kirsner RS, Eaglstein WH. The wound healing process. Dermatol Clin 1993; 11:629-640.
https://doi.org/10.1016/S0733-8635(18)30216-X
Falanga V, Martín TA, Tagaki H, et al. Low oxygen tension increases mRNA levels of a 1 procollagen in human dermal fibroblasts. J Cell Physiol 1993; 157:408-412.
https://doi.org/10.1002/jcp.1041570225
Xu J, Clark RA. Extracellular matrix alters PDGF regulation of fibroblast integrins. J Cell Biol 1996; 132:239-249.
https://doi.org/10.1083/jcb.132.1.239
Laiho M, Saksela O, Keski-Oja J. Transforming growth factor p induction of type-1 palasminogen activator inhibitor J Biol Chem 1987; 262:17467-17474.
Overall CM. lndependent regulation of collagenase, 72k0 progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor p. J Biol Chem 1989; 264:1860-1869.
Clark RA, Nielsen LO, Welch MP, et al. Collagen matrices attenuate the collagen synthetic response of cultured fibroblasts to TGF p. J Cell Sci 1995; 108:1251-1261.
Abercrombie M. Wound contraction in relation to collagen formation in scorbutic guinea pigs. J Embryol Exp Morphol 1956; 4:167-175.
Majno G, Gabbiani G. Contraction of granulation tissue in vitro: Similarity to smooth muscle. Science 1971; 173:548-550.
https://doi.org/10.1126/science.173.3996.548
Gabbiani G, Hirchel BJ, Ryan GB, et al. Granulation tissue as a contractile organ: a study of structure and function. J Exp Med 1972; 135:719-734.
https://doi.org/10.1084/jem.135.4.719
Risau W. Mechanism of angiogenesis. Nature 1997; 386:671-674.
https://doi.org/10.1038/386671a0
Maisonpiere PC, Suri C, Jons P, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277:55-60.
https://doi.org/10.1126/science.277.5322.55
Gailit J, Clark RA. Wound repair in the context of the extracellular matrix. Curr Opin Cell Biol 1994; 6:717-725.
https://doi.org/10.1016/0955-0674(94)90099-X
Bornstein P. Diversity of functions is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 1995; 130:503-506.
https://doi.org/10.1083/jcb.130.3.503
Sage EH. Pieces of eight: bioactive fragments of extracellular proteins as regulators of angiogenesis. Trends Cell Biol 1997; 7:182-186.
https://doi.org/10.1016/S0962-8924(97)01037-4
O'Reilly MS, Boehm T, Shing Y, et al. Endostatin, an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88:277-285.
https://doi.org/10.1016/S0092-8674(00)81848-6
Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin avP3 for angiogenesis. Science 1994; 264:569-571.
https://doi.org/10.1126/science.7512751
Tremble PM, Lane TF, Sage EH, et al. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol 1993;121:1433-1434.
https://doi.org/10.1083/jcb.121.6.1433
Scott FE. Proteoglycan-fibrillar collagen interactions in tissues: Dermatan sulfate proteoglycans a tissue organizer. En: JE Scott. Dermatan Sulfate Proteoglycans: Chemistry, Biology, Chemical Pathology, Londres 1993:165-181.
Yamagata M, Saga S, Kato M. Selective distributions of proteoglycans and their ligands in pericellular matrix of cultured fibroblasts: lmplications for their roles in cell-substratum adhesion. J Cell Sci 1993; 106:55-65.
Hering TM, Marchant RE, Andeson JM. Type V collagen during granulation tissue development. Exp Mol Pathol 1983; 39:219-229.
https://doi.org/10.1016/0014-4800(83)90053-9
Keene DR, Engvall E, Glanville RW. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous net-work. J Cell Biol 1988; 107:1995-2006.
https://doi.org/10.1083/jcb.107.5.1995
Postlewaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblast to type 1, 11, and 111 collagens and collagen-derived peptides. Proc Natl Acad Sci USA 1978; 75:871-875.
https://doi.org/10.1073/pnas.75.2.871
Hynes RO. lntegrins: Versatility, modulation and signalling in cell adhesión. Cell 1992; 69:11-25.
https://doi.org/10.1016/0092-8674(92)90115-S
Clark EA, Brugge JS. lntegrins and signal transduction pathways: The road taken. Science 1995; 268:233-239.
https://doi.org/10.1126/science.7716514
Lawrence WT, Oiegelmann RF. Growth Factors in Wound Healing. Clin Oermat 1994; 12:157-169.
https://doi.org/10.1016/0738-081X(94)90266-6
Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impared wound healing. J Clin lnvest 1997; 109:132-138.
https://doi.org/10.1111/1523-1747.ep12319188
Schwartz SI, Shires GT, Spencer FC, et al. Cuidado y cicatrizacióndelasheridas. En:CohenIK,Diegelmann RF, Crossland MC. Principios de Cirugía 1994:287-312.
F ailla CM, Odorisio T, Cianfarani F. Placenta! growth factor is induced in human queratinocites during wound healing. J Clin lnvest 2000; 115:388-395.
https://doi.org/10.1046/j.1523-1747.2000.00085.x
Mann AA, Breuhahn K, Schirmacher P, et al. Keratinocyte-derived G-Mac CSF accelerates wound healing. J Clin lnvest 2001; 117:1382-1390.
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






