Alopecia areata universalis successfully treated baricitinib
DOI:
https://doi.org/10.29176/2590843X.1824Keywords:
alopecia areata, Janus Kinase Inhibitors, BaricitinibAbstract
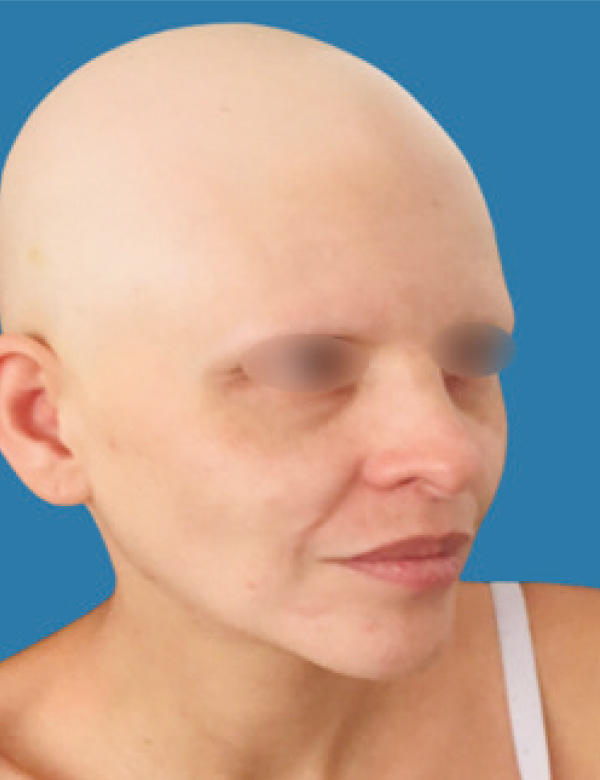
Alopecia areata is a dermatological disease characterized by hair loss of non-scarring nature, of autoimmune origin, which affects approximately 2% of the population [1,2]. It is a multifactorial disease, with genetic predisposition, associated with triggering factors (psychological and stress) and other immune-mediated diseases (thyroiditis, vitiligo).
Its most common clinical presentation is the appearance of one or more plaques of alopecia on the scalp, beard, eyebrows or eyelashes, well defined, with the traction sign present at the edges of lesions, in addition to hairs in "exclamation mark" on the alopecic area (1,2,4).
There is a wide variety of treatments, both local and systemic, however, ineffective in the long term and with high adverse risk when maintaining therapy. New treatment strategies have emerged such as selective janus kinase inhibitors, promising for the treatment of alopecia areata in the long term. We present below a case of alopecia areata universalis successfully treated with Baricitinib
Author Biographies
Manuel Darío Franco, Universidad del Bosque, Bogotá Colombia.
Médico Dermatólogo, Universidad del Bosque, Bogotá Colombia.
María José Giraldo Parra, Universidad del Rosario, Bogotá Colombia.
Médica General, Universidad del Rosario, Bogotá Colombia.
References
Sterkens A, Lambert J, Bervoets A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021 May;21(2):215-230. doi: 10.1007/s10238-020-00673-w. Epub 2021 Jan 1. PMID: 33386567.
Zhou C, Li X, Wang C, Zhang J. Alopecia Areata: an Update on Etiopathogenesis, Diagnosis, and Management. Clin Rev Allergy Immunol. 2021 Dec;61(3):403-423. doi: 10.1007/s12016-021-08883-0. Epub 2021 Aug 17. PMID: 34403083.
Teontor Simakou, John P. Butcher, Stuart Reid, Fiona L. Henriquez, Alopecia areata: A multifactorial autoimmune condition, Journal of Autoimmunity, Volume 98, 2019, Pages 74-85, ISSN 0896-8411 https://doi.org/10.1016/j.jaut.2018.12.001.
Brett King, Justin Ko, Seth Forman, Manabu Ohyama, Natasha Mesinkovska, Guanglei Yu, Jill McCollam, Margaret Gamalo, Jonathan Janes, Emily Edson-Heredia, Katrin Holzwarth, Yves Dutronc, Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: Phase 2 results from a randomized controlled study,Journal of the American Academy of Dermatology,Volume 85, Issue 4, 2021, Pages 847-853, ISSN 0190-9622, https://doi.org/10.1016/j.jaad.2021.05.050.
King B, Ohyama M, Kwon O, Zlotogorski A, Ko J, Mesinkovska NA, Hordinsky M, Dutronc Y, Wu WS, McCollam J, Chiasserini C, Yu G, Stanley S, Holzwarth K, DeLozier AM, Sinclair R; BRAVE-AA Investigators. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N Engl J Med. 2022 May 5;386(18):1687-1699. doi: 10.1056/NEJMoa2110343. Epub 2022 Mar 26. PMID: 35334197.
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Revista de la Asociación Colombiana de Dermatología y Cirugía Dermatológica

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.

| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






