Effectiveness and security of shampoo versus oral ivermectine in the treatment for pediculosis capitis
DOI:
https://doi.org/10.29176/2590843X.272Keywords:
Lice infectations, ivermectin, pediculus, public health, therapies, investigational, local symptomsAbstract
Introduction: Pediculosis capitis is a pubic health problem that may affect anyone regardless of race or economic level. The causative organism is Pediculus humanus capitis, an ectoparasite that lives on the hair and feeds on blood.
Objective: To compare the effectiveness and safety of ivermectine 0.1 %, shampoo with oral ivermectine 200 µg/kg for treatment of pediculosis capitis.
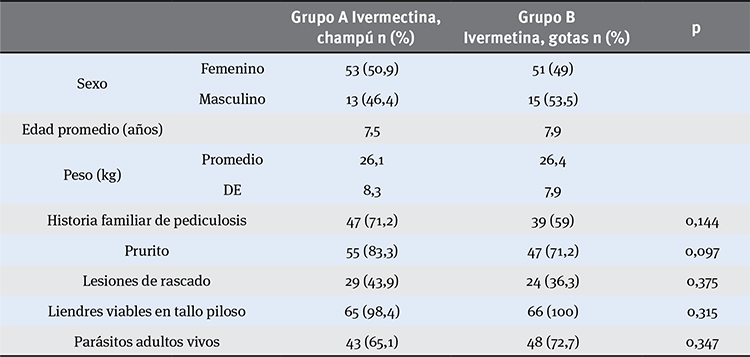
Materials and methods: The study was a randomized, double-blind clinical trial. In this study we analyzed 437 children and 132 met the inclusion criteria. People were divided in two groups. Group A received a topic treatment of 0.1 % ivermectine shampoo, unique doses of 400 µg/kg and oral drops of placebo. Group B received 0.6 % ivermectine drops, 200 µg/kg (1 drop/kg) and placebo shampoo.
Results: Patients were examined on the 7th and 14th day after starting treatment. Ivermectine shampoo had a one-dose cure rate of 87.8 % versus the oral treatment with 74.2 %, both after seven days. A second dose was used on seven days after the first dose and only in uncured cases and these increased the cure rate of 94.3 % and 91.9 % respectively.
Conclusions: The effectiveness and safety of the topical vehicle is similar to oral therapy and did not increase adverse effects of this new therapy.
Author Biographies
Christian Xavier Burbano
Médico dermatólogo, Universidad El Bosque, Bogotá, D.C., Colombia
John Fabio Zúñiga
Médico dermatólogo, Universidad El Bosque, Bogotá, D.C., Colombia
Adriana Motta
Médica dermatóloga, Departamento de Dermatología, Facultad de Medicina, Universidad El Bosque, Bogotá, D.C., Colombia
Hospital Simón Bolívar, Bogotá, D.C., Colombia
Luz Karem Morales
Hospital Simón Bolívar, Bogotá, D.C., Colombia
Médica, Universidad de La Sabana, Bogotá, D.C., Colombia
References
2. Malcolm C, Bergman J. Trying to keep ahead of lice: A therapeutic challenge. Skin Therapy Letter. 2006;11:1-6.
3. Downs AM, Stafford KA, Harvey I, Coles GC. Evidence for double resistance to permethrin and malathion in head lice. Br J Dermatol. 1999;141:508-11.
4. Meinking TL, Serrano L, Hard B, Bruce MA, Pamela JD, Glendene MA, et al. Comparative in vitro pediculicidal efficacy of treatments in a resistant head lice population in the United States. Arch Dermatol. 2002;138:220-4.
5. Victoria J, Trujillo R. Topical ivermectin: A new succesful treatment for scabies. Pediatric Dermatol. 2001;18:63-5.
6. Victoria J. Uso de ivermectina en niños. Dermatol Pediatr Let. 2003;1:61-5.
7. Comité Nacional de Dermatología Pediátrica. Pediculosis y escabiosis. Arch Argent Pediatr. 2001;99:69-74.
8. Schenone H, Weidmaier G, Contreras L. Tratamiento de la pediculosis capitis en niños con permetrina al 1% en champú o loción. Bol Chil Parasitol. 1994;49:49-52.
9. Piquero-Casals J, Piquero-Casals V, Pérez M, Quintero I, Ramírez B, Piquero-Martín J. Epidemiología de la pediculosis capitis en escolares del Distrito Sanitario No. 3 en Caracas, Venezuela. Revista Venezolana de Dermatología. 2004;42:19-22.
10. Glaziou P, Nyguyen LN, Moulia-Pelat JP, Cartel JL, Martin PM. Efficacy of ivermectin for the treatment of head lice. Trop Med Parasitol. 1994;45:253-4.
11. Youssef MY, Sadaka HA, El-Ariny AF. Topical application of ivermectin for human ectoparasites. Am J Trop Med Hyg. 1995;53:652-3.
12. U.S. Food and Drug Administration. FDA approval 02/07/2012. Department of Health and Human Services. Disponible en: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202736_sklice_toc.cfm. Consultado el 15 de julio 2014.
13. Victoria J, Ahumada NS, González F. Pediculosis capitis: tratamiento de 100 niños con ivermectina. Act Terap Dermatol.1997;20:99-103.
14. Victoria J. Ivermectina en pediculosis capitis. Act Terap Dermatol. 1998;21:448-51.
15. Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, et al. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol 2002;42:1122-33.
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section

| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






