Effectiveness of oral ketoconazole in the treatment of moderate to severe seborrheic dermatitis
Keywords:
ketoconazole, seborrheic dermatitis, effectiveness, dermatologyAbstract
Introduction: Seborrheic dermatitis is a chronic inflammatory disease difficult to treat that mainly affects seborrheic areas of skin and scalp. Successful therapy with imidazole derivatives like ketoconazole is variable depending on the severity of the lesions and the affected areas, as well as medication administration. Oral ketoconazole has been considered the first-line therapy for moderate to severe seborrheic dermatitis at the Centro Dermatológico Federico Lleras Acosta, nevertheless no clinical trials evaluating the action and effectiveness of ketoconazole in seborrheic dermatitis are available in Colombia. The aim of this study was to establish the effectiveness of oral ketoconazole in moderate to severe cases of seborrheic dermatitis by comparing clinical parameters before and after the treatment.
Materials and methods: A quasi-experimental design was used to evaluate effectiveness of ketoconazole at 200 mg once daily for 30 days. The study population included 96 patients with diagnosis of moderate to severe seborrheic dermatitis who accepted to participate in the study following the principles of good clinical practice. The seborrheic dermatitis severity scale of the Centro Dermatológico Federico Lleras Acosta clinical practice guideline was used to assess the grade of severity of seborrheic dermatitis. This scale includes scaling, itching, ertythema and anatomic location. The scores are set in ranges from 0 to 100, where the maximum level is 100. Patients with scores equal to or greater than 50 were included as a cut-off score to define moderate severity. Those who scored above 75 points were considered severe cases. General improvement degree was calculated from the difference between initial and final measure values. A treatment was considered effective when at least a 70% improvement was reached.
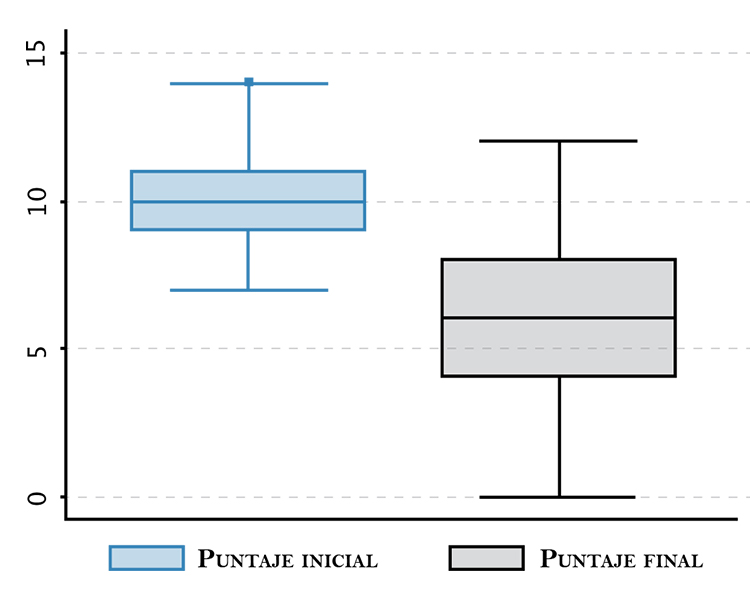
Results: Eighty-six patients finished the study, and 11% were lost to followup. Mean score was 67 points at start and 33 points at end. We found statistically significant differences between means before and after treatment (p-value: 0,001). Forty-eight percent of patients showed at least a 70% improvement. There were adverse effects in 11 patients (12,8 %; CI 95%: 6,5-21,7) all of them were minor and self-limiting.
Conclusion: Oral ketoconazole is a therapeutic alternative in the treatment for patients with moderate to severe seborrheic dermatitis. It has an adequate side effect profile at the doses used and during the time described in this study.
Author Biographies
Camilo Andrés Morales
Médico dermatólogo, Oficina de Docencia e Investigación, Centro Dermatológico Federico Lleras Acosta, E.S.E., Bogotá, D.C., Colombia
Guillermo Sánchez
Médico epidemiólogo, M.Sc. en Epidemiología Clínica, Oficina de Docencia e Investigación, Centro Dermatológico Federico Lleras Acosta, E.S.E., Bogotá, D.C., Colombia
References
2. Schwartz RA, Janusz CA, Janniger CK. Seborrheic dermatitis: an overview. Am Fam Physician. 2006;74:125-30.
3. Borgers M, Degreef H. The role of ketoconazole in seborrheic dermatitis. Cutis. 2007;80:359-63.
4. Gupta AK, Nicol K, Batra R. Role of antifungal agents in the treatment of seborrheic dermatitis. Am J Clin Dermatol. 2004;5:417-22.
5. Faergemann J, Borgers M, Degreef H. A new ketoconazole topical gel formulation in seborrhoeic dermatitis: an updated review of the mechanism. Expert Opin Pharmacother. 2007;8:1365-71.
6. Faergemann J. In vitro and in vivo activities of ketoconazole and itraconazole against Pityrosporum orbiculare. Antimicrob Agents Chemother. 1984;26:773-4.
7. Strippoli V, Piacentini A, D’Auria FD, Simonetti N. Antifungal activity of ketoconazole and other azoles against Malassezia furfur in vitro and in vivo. Infection. 1997;25:303-6.
8. van Cutsem J, van Gerven F, Cauwenbergh G, Odds F, Janssen PA. The antiinflammatory effects of ketoconazole. A comparative study with hydrocortisone acetate in a model using living and killed Staphylococcus aureus on the skin of guineapigs. J Am Acad Dermatol. 1991;25:257-61.
9. Hegemann L, Toso SM, Lahijani KI, Webster GF, Uitto J. Direct interaction of antifungal azole-derivatives with calmodulin: a possible mechanism for their therapeutic activity. J Invest Dermatol. 1993;100:343-6.
10. Farr PM, Krause LB, Marks JM, Shuster S. Response of scalp psoriasis to oral ketoconazole. Lancet. 1985;2:921-2.
11. Sohnle PG, Collins-Lech C. Activation of complement by Pityrosporum orbiculare. J Invest Dermatol. 1983;80:93-7.
12. Shuster S. The aetiology of dandruff and the mode of action of therapeutic agents. Br J Dermatol. 1984;111:235-42.
13. Ackerman AB, Kligman AM. Some observations on dandruff. J Soc Cosmet Chem. 1967;20:81-101.
14. Leyden JJ, McGinley KJ, Kligman AM. Role of microorganisms in dandruff. Arch Dermatol. 1976;112:333-8.
15. Alexander S. Do shampoos affect dandruff ? Br J Dermatol. 1967;79:92-5.
16. Aron-Brunetiere R, Dompmartin-Pernot D, Drouhet E. Treatment of pityriasis capitis (dandruff) with econazole nitrate. Acta Derm Venereol. 1977;57:77-80.
17. Ro BI, Dawson TL. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J Investig Dermatol Symp Proc. 2005;10:194-7.
18. Pierard-Franchimont C, Hermanns JF, Degreef H, Pierard GE. From axioms to new insights into dandruff. Dermatology. 2000;200:93-8.
19. Gupta AK, Bluhm R, Cooper EA, Summerbell RC, Batra R. Seborrheic dermatitis. Dermatol Clin. 2003;21:401-12.
20. Machin D, Campbell MJ. Statistical tables for the design of clinical trials. Oxford, St. Louis: Blackwell Scientific Publications; 1987.
21. Ministerio de Salud. Resolución N° 008430 de 1993: normas científicas, técnicas y administrativas para la investigación en salud; 1993. Fecha de consulta: 19 de julio de 2010. Disponible en: http://201.234.78.165:8080/portalcol/downloads/ archivosSoporteConvocatorias/751.pdf.
22. World Health Organization. WHO drug information. Current topics: Declaration of Helsinki and placebo-controlled clinical trials. Geneva: World Health Organization; 2001. Fecha de consulta: 19 de julio de 2010. Disponible en: http:// apps.who.int/medicinedocs/pdf/h2989e/h2989e.pdf.
23. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects; 2001. Fecha de consulta: 19 de julio de 2010. Disponible en: http://www.wma.net/ en/30publications/10policies/b3/17c.pdf.
24. McGrath J, Murphy GM. The control of seborrhoeic dermatitis and dandruff by antipityrosporal drugs. Drugs. 1991;41:178-84.
25. Faergemann J, Jones JC, Hettler O, Loria Y. Pityrosporum ovale (Malassezia furfur) as the causative agent of seborrhoeic dermatitis: new treatment options. Br J Dermatol. 1996;134:12-5.
26. Bergbrant IM. Seborrhoeic dermatitis and Pityrosporum yeasts. Curr Top Med Mycol. 1995;6:95-112.
27. Sei Y, Hamaguchi T, Ninomiya J, Nakabayashi A, Takiuchi I. Seborrhoeic dermatitis: treatment with anti-mycotic agents. J Dermatol. 1994;21:334-40.
28. van Cutsem J, van Gerven F, Fransen J, Schrooten P, Janssen PA. The in vitro antifungal activity of ketoconazole, zinc pyrithione, and selenium sulfide against Pityrosporum and their efficacy as a shampoo in the treatment of experimental pityrosporosis in guinea pigs. J Am Acad Dermatol. 1990;22:993-8.
29. Farr PM, Shuster S. Treatment of seborrhoeic dermatitis with topical ketoconazole. Lancet. 1984;2:1271-2.
30. Pierard-Franchimont C, Pierard GE, Arrese JE, De Doncker P. Effect of ketoconazole 1% and 2% shampoos on severe dandruff and seborrhoeic dermatitis: clinical, squamometric and mycological assessments. Dermatology. 2001;202:171-6.
31. Peter RU, Richarz-Barthauer U. Successful treatment and prophylaxis of scalp seborrhoeic dermatitis and dandruff with 2% ketoconazole shampoo: results of a multicentre, double-blind, placebo-controlled trial. Br J Dermatol. 1995;132:441-5.
32. Green CA, Farr PM, Shuster S. Treatment of seborrhoeic dermatitis with ketoconazole. II. Response of seborrhoeic dermatitis of the face, scalp and trunk to topical ketoconazole. Br J Dermatol. 1987;116:217-21.
33. Elewski B, Ling MR, Phillips TJ. Efficacy and safety of a new once-daily topical ketoconazole 2% gel in the treatment of seborrheic dermatitis: a phase III trial. J Drugs Dermatol. 2006;5:646-50.
34. Zienicke H, Korting HC, Braun-Falco O, Effendy I, Hagedorn M, Kuchmeister B, et al. Comparative efficacy and safety of bifonazole 1% cream and the corresponding base preparation in the treatment of seborrhoeic dermatitis. Mycoses. 1993;36:325-31.
35. Dupuy P, Maurette C, Amoric JC, Chosidow O. Randomized, placebo-controlled, double-blind study on clinical efficacy of ciclopiroxolamine 1% cream in facial seborrhoeic dermatitis. Br J Dermatol. 2001;144:1033-7.
36. Pierard-Franchimont C, Goffin V, Decroix J, Pierard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatitis. Skin Pharmacol Appl Skin Physiol. 2002;15:434-41.
37. Dreno B, Chosidow O, Revuz J, Moyse D. Lithium gluconate 8% vs ketoconazole 2% in the treatment of seborrhoeic dermatitis: a multicentre, randomized study. Br J Dermatol. 2003;148:1230-6.
38. Chosidow O, Maurette C, Dupuy P. Randomized, openlabeled, non-inferiority study between ciclopiroxolamine 1% cream and ketoconazole 2% foaming gel in mild to moderate facial seborrheic dermatitis. Dermatology. 2003;206:233-40.
39. Shuster S, Meynadier J, Kerl H, Nolting S. Treatment and prophylaxis of seborrheic dermatitis of the scalp with antipityrosporal 1% ciclopirox shampoo. Arch Dermatol. 2005;141:47-52.
40. Elewski BE, Abramovits W, Kempers S, Schlessinger J, Rosen T, Gupta AK, et al. A novel foam formulation of ketoconazole 2% for the treatment of seborrheic dermatitis on multiple body regions. J Drugs Dermatol. 2007;6:1001-8.
41. Ford GP, Farr PM, Ive FA, Shuster S. The response of seborrhoeic dermatitis to ketoconazole. Br J Dermatol. 1984;111:603-7.
42. Hjorth N, Clemmensen OJ. Treatment of dermatitis of the head and neck with ketoconazole in patients with type I hypersensitivity for Pityrosporum orbiculare. Semin Dermatol. 1983;2:26-9.
43. Rosenberg EW, Belew PW. Improvement of psoriasis of the scalp with ketoconazole. Arch Dermatol. 1982;118:370-1.
44. Wishner AJ, Teplitz ED, Goodman DS. Pityrosporum, ketoconazole, and seborrheic dermatitis. J Am Acad Dermatol. 1987;17:140-1.
45. Baysal V, Yildirim M, Ozcanli C, Ceyhan AM. Itraconazole in the treatment of seborrheic dermatitis: a new treatment modality. Int J Dermatol. 2004;43:63-6.
46. Vena GA, Micali G, Santoianni P, Cassano N, Peruzzi E. Oral terbinafine in the treatment of multi-site seborrhoic dermatitis: a multicenter, double-blind placebo-controlled study. Int J Immunopathol Pharmacol. 2005;18:745-53.
47. Kose O, Erbil H, Gur AR. Oral itraconazole for the treatment of seborrhoeic dermatitis: an open, noncomparative trial. J Eur Acad Dermatol Venereol. 2005;19:172-5.
48. Zisova LG. Fluconazole and its place in the treatment of seborrheic dermatitis-new therapeutic possibilities. Folia Med (Plovdiv). 2006;48:39-45.
49. Pierard GE, Ausma J, Henry F, Vroome V, Wouters L, Borgers M, et al. A pilot study on seborrheic dermatitis using pramiconazole as a potent oral anti-Malassezia agent. Dermatology. 2007;214:162-9.
50. Shemer A, Kaplan B, Nathansohn N, Grunwald MH, Amichai B, Trau H. Treatment of moderate to severe facial seborrheic dermatitis with itraconazole: an open non-comparative study. Isr Med Assoc J. 2008;10:417-8.
51. Park HS, Chong HW, Lee YW, Huh CH, Lee HI, Kim BJ, Kim MN. A study upon parameters useful for evaluating the antidandruff efficacy of 1% zinc pyrithione shampoo. Int J Trichol. 2009;1:60-1.
52. Leyden JJ MK, Kligman AM. Shorter methods for evaluating antidandruff agents. J Soc Cosmet Chem. 1975;26:573-80.
53. Piérard Franchimont C, Uhoda E, Loussouarn G, Saint Léger D, Piérard G. Effect of residence time on the efficacy of antidandruff shampoos. Int J Cosmet Sci. 2003;25:267-71.
54. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy. Analysis of 33 cases. Gastroenterology. 1984;86:503-13.
55. Janssen PA, Symoens JE. Hepatic reactions during ketoconazole treatment. Am J Med. 1983;74:80-5.
56. Stricker BH, Blok AP, Bronkhorst FB, van Parys GE, Desmet VJ. Ketoconazole-associated hepatic injury. A clinicopathological study of 55 cases. J Hepatol. 1986;3:399-406. 5
7. Bercoff E, Bernuau J, Degott C, Kalis B, Lemaire A, Tilly H, et al. Ketoconazole-induced fulminant hepatitis. Gut. 1985;26:636-8.
58. Findor JA, Sorda JA, Igartua EB, Avagnina A. Ketoconazoleinduced liver damage. Medicina (B Aires). 1998;58:277-81.
59. Chien RN, Sheen IS, Liaw YF. Unintentional rechallenge resulting in a causative relationship between ketoconazole and acute liver injury. Int J Clin Pract. 2003;57:829-30.
60. van Parys G, Evenepoel C, van Damme B, Desmet VJ. Ketoconazole-induced hepatitis: a case with a definite cause-effect relationship. Liver. 1987;7:27-30.
61. Duarte PA, Chow CC, Simmons F, Ruskin J. Fatal hepatitis associated with ketoconazole therapy. Arch Intern Med. 1984;144:1069-70.
62. Klausner MA. Ketoconazole and hepatitis. J Am Acad Dermatol. 1992;26:1028-30.
63. Garcia-Rodriguez LA, Duque A, Castellsague J, PerezGutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48:847-52.
64. Chien RN, Yang LJ, Lin PY, Liaw YF. Hepatic injury during ketoconazole therapy in patients with onychomycosis: a controlled cohort study. Hepatology. 1997;25:103-7.
How to Cite
Downloads
Downloads
Published
How to Cite
Issue
Section
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |






