Criopirinopatías: ¿qué son?, ¿cómo detectarlas? y ¿cuál es el enfoque de su tratamiento?
DOI:
https://doi.org/10.29176/2590843X.983Palabras clave:
síndromes periódicos asociados con criopirinas, síndrome CINCA, síndrome articular y cutáneo neurológico infantil crónico, criopirinopatía, síndrome autoinflamatorio familiar por frío, urticaria familiar por frío, enfermedad inflamatoria multisistémica de inicio en la infancia (IOMID), síndrome de Muckle-Wells, enfermedad inflamatoria multisistémica de inicio en el periodo neonatal (NOMID), proteína con dominio pirina 3 de la familia NLRResumen
Los síndromes periódicos asociados con criopirinas (Cryopyrin Associated Periodic Syndromes, CAPS), o criopirinopatías, son enfermedades raras que hacen parte de los síndromes autoinflamatorios, caracterizados por episodios de fiebre recurrente e inflamación sin etiología autoinmunitaria, neoplásica ni infecciosa. Entre estos síndromes, los CAPS incluyen tres entidades: el síndrome autoinflamatorio familiar por frío (Familial Cold Autoinflammatory Syndrome, FCAS), el síndrome de Muckle-Wells y la enfermedad multisistémica inflamatoria de inicio neonatal (Neonatal Onset Multisystem Inflammatory Disease, NOMID), también conocida como síndrome articular, cutáneo y neurológico crónico infantil (Chronic Infantile Neurological Cutaneous and Articular (CINCA) syndrome).
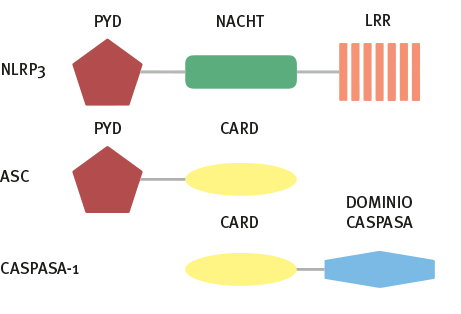
Se han identificado mutaciones en el gen NLRP3 como etiología de estos síndromes, con un patrón de herencia autosómico dominante en la mayoría de los casos. El diagnóstico suele hacerse por sus manifestaciones clínicas, con apoyo de la biopsia de piel, y se confirma con el estudio genético. Hasta la fecha, el tratamiento basado en el bloqueo de la IL-1β, ha mostrado una mejoría satisfactoria en la mayoría de los pacientes.
Biografía del autor/a
Maribel Gallego
Médico, dermatólogo, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Catalina Jaramillo
Médico, dermatólogo, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Jaime Sierra
Médico, dermatólogo, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Margarita María Velásquez
Médica dermatóloga, doctora en Ciencias Básicas Biomédicas con énfasis en Inmunología; profesora, Sección de Dermatología, Centro de Investigaciones Dermatológicas CIDERM, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Referencias bibliográficas
Neven B, Prieur A-M, Quartier dit Maire P. Cr¬yopyrinopathies: Update on pathogenesis and treatment. Nat Clin Pract Rheumatol. 2008;4:481-9.
https://doi.org/10.1038/ncprheum0874
Aróstegui JI. Hereditary systemic autoinflamma¬tory diseases. Reumatol Clin. 2011;7:45-50.
https://doi.org/10.1016/j.reuma.2010.01.010
McDermott MF, Aksentijevich I, Galon J, McDer¬mott EM, Ogunkolade BW, Centola M, et al. Ger¬mline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syn¬dromes. Cell. 1999;97:133-44.
https://doi.org/10.1016/S0092-8674(00)80721-7
Sánchez G a M, de Jesús AA, Goldbach-Mansky R. Monogenic autoinflammatory diseases: Disorders of amplified danger sensing and cytokine dysregu¬lation. Rheum Dis Clin North Am. 2013;39:701-34.
https://doi.org/10.1016/j.rdc.2013.08.001
Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55:101-5.
https://doi.org/10.1536/ihj.13-388
Chai J, Shi Y. Apoptosome and inflammasome: Conserved machineries for caspase activation. Natl Sci Rev. 2014;1:101-18.
https://doi.org/10.1093/nsr/nwt025
Aróstegui JI. Etiopatogenia de los síndromes aso¬ciados a criopirina: genética, bases moleculares y el inflamasoma. Med Clin. 2011;136(Supl.1):22-8.
https://doi.org/10.1016/S0025-7753(11)70005-7
Ahmadi N, Brewer CC, Zalewski C, King KA, Butman JA, Plass N, et al. Cryopyrin-associated periodic syndromes: Otolaryngologic and audio¬logic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302.
https://doi.org/10.1177/0194599811402296
Rojo E. Síndromes de fiebre recurrente y su rele¬vancia en la medicina contemporánea. Acta Mé¬dica Grup Ángeles. 2009;7:150-7.
Jiménez S. De la fiebre periódica a los sín¬dromes autoinflamatorios. Boletín de Pediatría. 2011;51:194-203.
Pérez B, Díaz M, Sexto L. Alergia, urticaria de con¬tacto y síndromes urticariformes inducidos por frío. Galicia Clínica. 2012;73:151-9.
https://doi.org/10.22546/21/398
Vergara C. Síndromes autoinflamatorios. Rev Chil Reumatol. 2008;24:206-11.
Almeida de Jesús A, Goldbach-Mansky R. Mono¬genic autoinflammatory diseases: Concept and cli¬nical manifestations. Clin Immunol. 2013;147:155-74.
https://doi.org/10.1016/j.clim.2013.03.016
Aboín-González S, Aldanondo-Fernández de la Mora I, García-Acebes ER, Carillo-Gijón R, Harto-Castaño A, Jaén-Olasolo P. Exacerbation of skin lesions during fever in a patient with chronic in¬fantile neurologic cutaneous articular syndrome. Actas Dermosifiliogr. 2008;99:481-4.
https://doi.org/10.1016/S0001-7310(08)74721-5
Yu JR, Leslie KS. Cryopyrin-associated periodic syndrome: An update on diagnosis and treatment response. Curr Allergy Asthma Rep. 2011;11:12-20.
https://doi.org/10.1007/s11882-010-0160-9
Gattorno M, Federici S, Pelagatti MA, Caorsi R, Brisca G, Malattia C, et al. Diagnosis and manage¬ment of autoinflammatory diseases in childhood. J Clin Immunol. 2008;28(Suppl.1):S73-83.
https://doi.org/10.1007/s10875-008-9178-3
Bolaños L, Mosquera-Reboredo JM, Cao M, Fe¬rreiro T, Veleiro B, Valdés F, et al. Renal and thyroid amyloidosis secondary tocryopyrin-associated pe¬riodic syndrome (Muckle-Wells syndrome) (NLRP3 mutation). Nefrologia. 2013;33:266-71.
Penadés IC, Montesinos BL, Puche AM. Sín¬drome de Muckle-Wells y síndrome autoinfla¬matorio familiar inducido por frío. Med Clin. 2011;136(Supl.1):16-21.
https://doi.org/10.1016/S0025-7753(11)70004-5
Federici S, Gattorno M. A practical approach to the diagnosis of autoinflammatory diseases in child¬hood. Best Pract Res Clin Rheumatol. 2014;28:263-76.
https://doi.org/10.1016/j.berh.2014.05.005
Furr JC, Panda M. Cold-induced urticaria with a familial transmission: a case report and review of the literature. J Med Case Rep. 2012;6:70.
https://doi.org/10.1186/1752-1947-6-70
Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: Phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol. 2001;108:615-20.
https://doi.org/10.1067/mai.2001.118790
Johnstone R. A large kindred with familial cold autoinflammatory syndrome. Ann Alergy Asthma Immunol. 2003;90:233-7.
https://doi.org/10.1016/S1081-1206(10)62147-3
Kuemmerle-Deschner JB, Lohse P, Koetter I, Dan¬necker GE, Reess F, Ummenhofer K, et al. NLRP3 E311K mutation in a large family with Muckle-Wells syndrome--description of a heterogeneous phenotype and response to treatment. Arthritis Res Ther. 2011;13:R196.
https://doi.org/10.1186/ar3526
Dávila-Seijo P, Hernández-Martín A, Torrelo A. Au¬toinflammatory syndromes for the dermatologist. Clin Dermatol. 2014;32:488-501.
https://doi.org/10.1016/j.clindermatol.2014.02.004
Moreno S, Buestán A, Véliz C, Mora C, González S, Down D, et al. Síndromes autoinflamatorios para el dermatólogo. Dermatología Pediátrica Latinoa¬mericana. 2007;5:9-18.
Hinojosa-Azaola A, Alcocer-Varela J. Enferme¬dades autoinflamatorias: una mirada a la inmu¬nidad innata y su patología. Rev Investig Clin. 2012;64:477-86.
Calvo Ry C, Soler-Palacín P, Merino R, Saavedra J, Antón J, Aróstegui JI, et al. Consensus document on the differential diagnosis and therapeutic ap¬proach to recurrent fever by the Paediatric Infec¬tology Society and the Paediatric Rheumatology Society. An Pediatr (Barc). 2011;74:194.e1-16.
https://doi.org/10.1016/j.anpedi.2010.09.022
Rowczenio DM, Trojer H, Russell T, Baginska A, Lane T, Stewart NM, et al. Clinical characteris¬tics in subjects with NLRP3 V198M diagnosed at a single UK center and a review of the literature. Arthritis Res Ther. 2013;15:R30.
https://doi.org/10.1186/ar4171
Tanaka N, Izawa K, Saito MK, Sakuma M, Oshima K, Ohara O, et al. High incidence of NLRP3 somatic mosaicism in patients with chronic infantile neu¬rologic, cutaneous, articular syndrome: Results of an International Multicenter Collaborative Study. Arthritis Rheum. 2011;63:3625-32.
https://doi.org/10.1002/art.30512
Izawa K, Hijikata A, Tanaka N, Kawai T, Saito MK, Goldbach-Mansky R, et al. Detection of base substitution-type somatic mosaicism of the NLRP3 gene with >99.9% statistical confidence by massi¬vely parallel sequencing. DNA Res. 2012;19:143-52.
https://doi.org/10.1093/dnares/dsr047
Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene enco¬ding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301-5.
Feldmann J, Prieur A-M, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurolo¬gical cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198-203.
https://doi.org/10.1086/341357
Martinon F, Burns K, Tschopp J. The inflam¬masome: A molecular platform triggering activa¬tion of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-26.
https://doi.org/10.1016/S1097-2765(02)00599-3
Tschopp J, Martinon F, Burns K. NALPs: A novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95-104.
https://doi.org/10.1038/nrm1019
Levy R, Gérard L, Kuemmerle-Deschner J, Lach¬mann HJ, Koné-Paut I, Cantarini L, et al. Pheno¬typic and genotypic characteristics of cryopyrin-associated periodic syndrome: A series of 136 patients from the Eurofever Registry. Ann Rheum Dis. 2014;18:1-7.
https://doi.org/10.1136/annrheumdis-2013-204991
Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. Inflammasome com¬ponents NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443-52.
https://doi.org/10.1369/jhc.6A7101.2006
Martinon F, Boveresses C, Epalinges C-. Inflam¬matory caspases spasessn F, Bintracellular innate immune system to autoinflammatory diseases cas¬pases not only play an essential role during apop¬totic. Cell Press. 2004;117:561-74.
https://doi.org/10.1016/j.cell.2004.05.004
Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ. The CARD plays a critical role in ASC foci forma¬tion and inflammasome signalling. Biochem J. 2013;449:613-21.
https://doi.org/10.1042/BJ20121198
Shi Y. Caspase activation: Revisiting the induced proximity model. Cell. 2004;117:855-8.
https://doi.org/10.1016/j.cell.2004.06.007
Nasti T. Inflammasome activation of IL-1 family mediators in response to cutaneous photodamage. Photochem Photobiol. 2012;88:1111-25.
https://doi.org/10.1111/j.1751-1097.2012.01182.x
Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137-61.
https://doi.org/10.1146/annurev-cellbio-101011-155745
Goldbach-Mansky R. Immunology in clinic review series; focus on autoinflammatory diseases: Up¬date on monogenic autoinflammatory diseases: The role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin Exp Immunol. 2012;167:391-404.
https://doi.org/10.1111/j.1365-2249.2011.04533.x
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflam¬masome activation targets caspase-11. Nature. 2011;479:117-21.
https://doi.org/10.1038/nature10558
Viganò E, Mortellaro A. Caspase-11: The driving factor for noncanonical inflammasomes. Eur J Im¬munol. 2013;43:2240-5.
https://doi.org/10.1002/eji.201343800
Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82-95.
https://doi.org/10.1111/nyas.12458
Gross O, Thomas CJ, Guarda G, Tschopp J. The in¬flammasome: An integrated view. Immunol Rev. 2011;243:136-51.
https://doi.org/10.1111/j.1600-065X.2011.01046.x
Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269-73.
https://doi.org/10.1038/nature08100
Hernández-Cuéllar E, Tsuchiya K, Hara H, Fang R, Sakai S, Kawamura I, et al. Cutting edge: Nitric oxide inhibits the NLRP3 inflammasome. J Im¬munol. 2012;189:5113-7.
https://doi.org/10.4049/jimmunol.1202479
Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflam¬masome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175-81.
https://doi.org/10.4049/jimmunol.1201516
Aksentijevich I, Putnam CD, Remmers EF, Mue¬ller JL, Le J, Kolodner RD, et al. The Clinical con¬tinuum of cryopyrinopathies novel CIAS1 mu¬tations in North American patients and a new cryopyrin model. Arthritis Rheumatol (Hoboken, NJ). 2007;56:1273-85.
https://doi.org/10.1002/art.22491
Razmara M, Srinivasula SM, Wang L, Poyet J, Geddes BJ, Distefano PS, et al. Mechanisms of signal transduction ECHANISMSprotein , a new CARD family member that regulates caspase-1 acti¬vation and apoptosis. J Biol Chem. 2002;277:13952-8.
https://doi.org/10.1074/jbc.M107811200
von Kampen O, Lipinski S, Till A, Martin SJ, Niet¬feld W, Lehrach H, et al. Caspase recruitment domain-containing protein 8 (CARD8) negatively regulates NOD2-mediated signaling. J Biol Chem. 2010;285:19921-6.
https://doi.org/10.1074/jbc.M110.127480
Ito S, Hara Y, Kubota T. CARD8 is a negative regu¬lator for NLRP3 inflammasome, but mutant NLRP3 in cryopyrin-associated periodic syndromes es¬capes the restriction. Arthritis Res Ther. 2014;16:1-11.
https://doi.org/10.1186/ar4483
Rubartelli A. Redox control of NLRP3 inflam¬masome activation in health and disease. J Leukoc Biol. 2012;92:951-8.
https://doi.org/10.1189/jlb.0512265
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem. 2014;395:203-30.
https://doi.org/10.1515/hsz-2013-0241
Dinarrello CA. Overview of the interleukin-1 fa¬mily of ligands and receptors. Immunology. 2013;25:389-93.
https://doi.org/10.1016/j.smim.2013.10.001
Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519-50.
https://doi.org/10.1146/annurev.immunol.021908.132612
Neill LAJO, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: Crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206-9.
https://doi.org/10.1016/S0167-5699(00)01611-X
Agostini L, Martinon F, Burns K, Mcdermott MF, Hawkins PN, Boveresses C, et al. NALP3 forms an IL-1 NL - processing inflammasome with increased activity in Muckle-Wells autoinflammatory di¬sorder. Immunity. 2004;20:319-25.
https://doi.org/10.1016/S1074-7613(04)00046-9
Vélez-Castrillón S, Camargo JF, Correa PA, Anaya J. Bases moleculares de la familia de la interleu¬quina-1. Rev Colomb Reumatol. 2004;11: 11-39.
Garlanda C, Riva F, Bonavita E, Mantovani A. Negative regulatory receptors of the IL-1 family. Semin Immunol. 2013;25:408-15.
https://doi.org/10.1016/j.smim.2013.10.019
Manzur F, Moneriz C. Canakinumab: un anti¬cuerpo monoclonal prometedor en el tratamiento de enfermedades cardiovasculares. Rev Colomb Cardiol. 2012;20:33-9.
https://doi.org/10.1016/S0120-5633(13)70022-6
Dinarello CA, Novick D, Kim S, Kaplanski G. In¬terleukin-18 and IL-18 binding protein. Front Im¬munol. 2013;4:289.
https://doi.org/10.3389/fimmu.2013.00289
Smith DE. The biological paths of IL-1 family mem¬bers IL-18 and IL-33. J Leukoc Biol. 2011;89:383-92.
https://doi.org/10.1189/jlb.0810470
Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123:4695-705.
https://doi.org/10.1172/JCI71543
Kakkar R, Lee RT. The IL-33/ST2 pathway: Thera¬peutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827-40.
https://doi.org/10.1038/nrd2660
Martin MU. Special aspects of interleukin-33 and the IL-33 receptor complex. Semin Immunol. Else¬vier Ltd; 2013;25:449-57.
https://doi.org/10.1016/j.smim.2013.10.006
Jimenez-Treviño S, Ramos-Polo E. Tratamiento de los síndromes periódicos asociados a criopirina (CAPS). Med Clin. 2011;136(Sup.1):29-33.
https://doi.org/10.1016/S0025-7753(11)70006-9
Doherty TA, Brydges SD, Hoffman HM. Autoin¬flammation: Translating mechanism to therapy. J Leukoc Biol. 2011;90:37-47.
https://doi.org/10.1189/jlb.1110616
Bachove I, Chang C. Anakinra and related drugs targeting interleukin-1 in the treatment of cryo¬pyrin- associated periodic syndromes. Open Ac-cess Rheumatol Res Rev. 2014;6:15-25.
https://doi.org/10.2147/OARRR.S46017
Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, Couloignier V, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem in¬flammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2010;62:258-67.
https://doi.org/10.1002/art.25057
Federici S, Martini A, Gattorno M. The central role of anti-IL-1 blockade in the treatment of mo¬nogenic and multi-factorial autoinflammatory di-seases. Front Immunol. 2013;4:351.
https://doi.org/10.3389/fimmu.2013.00351
Kuemmerle-Deschner JB, Tyrrell PN, Koetter I, Wittkowski H, Bialkowski A, Tzaribachev N, et al. Efficacy and safety of anakinra therapy in pe¬diatric and adult patients with the autoinflamma¬tory Muckle-Wells syndrome. Arthritis Rheum. 2011;63:840-9.
https://doi.org/10.1002/art.30149
Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607-12.
https://doi.org/10.1002/art.20033
Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N Engl J Med. 2006;581-92.
Lepore L, Paloni G, Caorsi R, Alessio M, Rigante D, Ruperto N, et al. Follow-up and quality of life of patients with cryopyrin-associated periodic syndromes treated with Anakinra. J Pediatr. 2010;157:310-5.e1.
https://doi.org/10.1016/j.jpeds.2010.02.040
Gillespie J, Mathews R, McDermott MF. Rilonacept in the management of cryopyrin-associated pe¬riodic syndromes (CAPS). J Inflamm Res. 2010;3:18.
Galeotti C, Koné-Paut I. Current options for the treatment of cryopyrin-associated periodic syn¬dromes. Expert Opin Orphan Drugs. 2013;1:589-97.
https://doi.org/10.1517/21678707.2013.821946
Goldbach-Mansky R, Shroff SD, Wilson M, Snyder C, Plehn S, Barham B, et al. A pilot study to eva¬luate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 TRAP) in patients with familial cold autoinflam¬matory syndrome. Arthritis Rheum. 2008;58:2432-42.
https://doi.org/10.1002/art.23620
Hoffman HM, Throne ML, Amar NJ, Sebai M, Ki¬vitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: Results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443-52.
https://doi.org/10.1002/art.23687
Hoffman HM, Throne ML, Amar NJ, Cartwright RC, Kivitz AJ, Soo Y, et al. Long-term efficacy and sa¬fety profile of rilonacept in the treatment of cryo¬pryin-associated periodic syndromes: Results of a 72-week open-label extension study. Clin Ther. 2012;34:2091-103.
https://doi.org/10.1016/j.clinthera.2012.09.009
Carné X. Canakinumab, un anticuerpo mono¬clonal frente a IL-1β, con utilidad potencial en distintos procesos inflamatorios. Med Clin. 2011;136(Supl.1):34-7.
https://doi.org/10.1016/S0025-7753(11)70007-0
Dhimolea E. Interleukin-1β inhibitors for the treatment of cryopyrin-associated periodic syn¬drome. Appl Clin Genet. 2011;4:21-7.
https://doi.org/10.2147/TACG.S8146
Hachulla E, Quartier P, Gitton X, Widmer A, et al. Use of canakinumab in the cryopyrin- associated periodic syndrome. N Engl J Med. 2009;360:2416-25.
https://doi.org/10.1056/NEJMoa0810787
Koné-Paut I, Lachmann HJ, Kuemmerle-Des¬chner JB, Hachulla E, Leslie KS, Mouy R, et al. Sustained remission of symptoms and improved health-related quality of life in patients with cryo¬pyrin-associated periodic syndrome treated with canakinumab: Results of a double-blind placebo-controlled randomized withdrawal study. Arthritis Res Ther. 2011;13:R202.
https://doi.org/10.1186/ar3535
Ter Haar N, Lachmann H, Özen S, Woo P, Uziel Y, Modesto C, et al. Treatment of autoinflammatory diseases: Results from the Eurofever Registry and a literature review. Ann Rheum Dis. 2013;72:678-85.
https://doi.org/10.1136/annrheumdis-2011-201268
Yokota S, Imagawa T, Nishikomori R, Takada H, Abrams K, Lheritier K, et al. Long-term safety and efficacy of canakinumab in cryopyrin-associated periodic syndrome: Results from an open-label, phase III pivotal study in Japanese patients.Clin Exp Rheumatol. 2017;35(Suppl.10):19-26.
Cómo citar
Descargas
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2019 Revista de la Asociación Colombiana de Dermatología y Cirugía Dermatológica

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.

| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |






