Papel de las quimiocinas en la inmunopatogénesis del linfoma cutáneo de células T
DOI:
https://doi.org/10.29176/2590843X.254Palabras clave:
linfoma cutáneo de células T, quimiocinas, CCL17, CCL22, CCR4, CXCR4Resumen
Los linfomas cutáneos de células T representan una expansión maligna de linfocitos de memoria que muestran una gran predilección por la piel. Son enfermedades de curso largo e indolente que pueden afectar la calidad de vida de forma significativa.
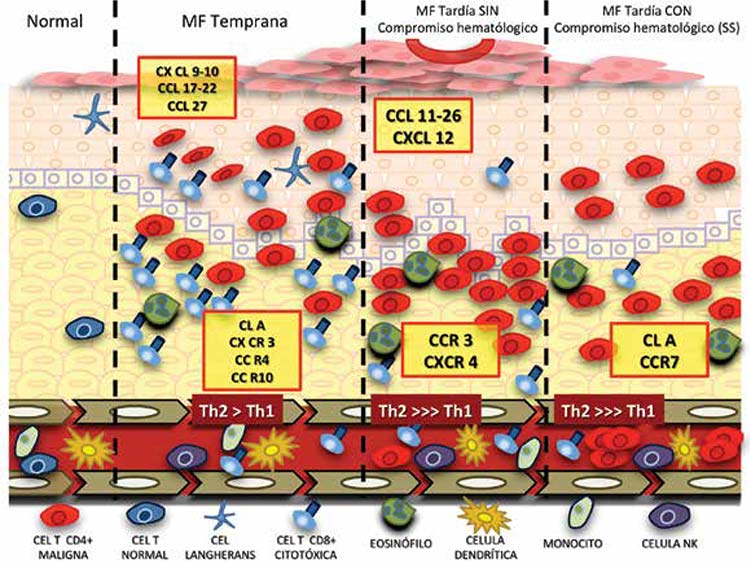
Las quimiocinas son una gran familia de citocinas relacionadas con la quimiotaxis, supervivencia y localización celular. Actúan sobre sus células blanco mediante receptores acoplados a proteínas G. En esta revisión se trata el papel que juegan las quimiocinas en el asentamiento de las células tumorales en la piel y su posterior relación con la infiltración de otros órganos; además; sobre cómo ellas son un posible blanco terapéutico para el manejo de estas enfermedades.
Biografía del autor/a
María Natalia Mejía
Médica, residente de segundo año de Dermatología, Sección de Dermatología, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Margarita María Velásquez
Médica dermatóloga, doctora en Ciencias Básicas Biomédicas con énfasis en Inmunología; profesora, Sección de Dermatología, Facultad de Medicina, Universidad de Antioquia; Centro de Investigaciones Dermatológicas, CIDERM, Medellín, Colombia
Referencias bibliográficas
2. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875-83.
3. Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032-43.
4. Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: Non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92:1240-51.
5. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005; 105:3768-85.
6. Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sèzary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-22.
7. Humme D, Lukowsky A, Sterry W. Diagnostic tools in mycosis fungoides. G Ital Dermatol Venereol. 2010;145:375-84.
8. Wood GS, Greenberg HL. Diagnosis, staging, and monitoring of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16:269-75.
9. Momtaz P, Zippin JH. Cutaneous T-cell lymphoma: A review of current therapies and the future therapeutic implications of chemokine biology. J Drugs Dermatol. 2009;8:1142-9.
10. Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sèzary syndrome. Lancet. 2008;371:945-57.
11. Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: Structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963-9.
12. Lu D, Duvic M, Medeiros LJ, Luthra R, Dorfman DM, Jones D. The T-cell chemokine receptor CXCR3 is expressed highly in low-grade mycosis fungoides. Am J Clin Pathol. 2001;115:413-21.
13. Kallinich T, Muche JM, Qin S, Sterry W, Audring H, Kroczek RA. Chemokine receptor expression on neoplastic and reactive T cells in the skin at different stages of mycosis fungoides. J Invest Dermatol. 2003;121:1045-52.
14. Sarris AH, Esgleyes-Ribot T, Crow M, Broxmeyer HE, Karasavvas N, Pugh W, et al. Cytokine loops involving interferon-gamma and IP-10, a cytokine chemotactic for CD4+ lymphocytes: An explanation for the epidermotropism of cutaneous T-cell lymphoma? Blood. 1995;86:651-8.
15. Tensen CP, Vermeer MH, van der Stoop PM, van Beek P, Scheper RJ, Boorsma DM, et al. Epidermal interferon-gamma inducible protein-10 (IP-10) and monokine induced by gamma-interferon (Mig) but not IL-8 mRNA expression is associated with epidermotropism in cutaneous T cell lymphomas. J Invest Dermatol. 1998;111:222-6.
16. Sugaya M. Chemokines and cutaneous lymphoma. J Dermatol Sci. 2010;59:81-5.
17. Wu CS, Wang ST, Liao CY, Wu MT. Differential CCR4 expression and function in cutaneous T-cell lymphoma cell lines. Kaohsiung J Med Sci. 2008;24:577-90.
18. Yamaguchi T, Ohshima K, Karube K, Kawano R, Nakayama J, Suzumiya J, et al. Expression of chemokines and chemokine receptors in cutaneous CD30+ lymphoproliferative disorders. Br J Dermatol. 2006;154:904-9.
19. Kakinuma T, Sugaya M, Nakamura K, Kaneko F, Wakugawa M, Matsushima K, et al. Thymus and activation-regulated chemokine (TARC/CCL17) in mycosis fungoides: Serum TARC levels reflect the disease activity of mycosis fungoides. J Am Acad Dermatol. 2003;48:23-30.
20. Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, et al. Circulating clonal CLA(+) and CD4(+) T cells in Sèzary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol. 2005;152:258-64.
21. Richardson SK, Newton SB, Bach TL, Budgin JB, Benoit BM, Lin JH, et al. Bexarotene blunts malignant T-cell chemotaxis in Sèzary syndrome: Reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-7.
22. Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157-65.
23. Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci. 1999;96:14470-5.
24. Capriotti E, Vonderheid EC, Thoburn CJ, Bright EC, Hess AD. Chemokine receptor expression by leukemic T cells of cutaneous T-cell lymphoma: Clinical and histopathological correlations. J Invest Dermatol. 2007;127:2882-92.
25. Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, et al. CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer. 2005;115:641-7.
26. Fujita Y, Abe R, Sasaki M, Honda A, Furuichi M, Asano Y, et al. Presence of circulating CCR10+ T cells and elevated serum CTACK/ CCL27 in the early stage of mycosis fungoides. Clin Cancer Res. 2006;12:2670-5.
27. Kagami S, Sugaya M, Minatani Y, Ohmatsu H, Kakinuma T, Fujita H, et al. Elevated serum CTACK/CCL27 levels in CTCL. J Invest Dermatol. 2006;126:1189-91.
28. Masui Y, Sugaya M, Kagami S, Fujita H, Yano S, Nagao M, et al. Sèzary syndrome treated with narrowband ultraviolet B: Timecourse measurement of serum levels of CCL17/CCL27. Clin Exp Dermatol. 2007;32:57-9.
29. Hoeller C, Richardson SK, Ng LG, Valero T, Wysocka M, Rook AH, et al. In vivo imaging of cutaneous T-cell lymphoma migration to the skin. Cancer Res. 2009;69:2704-8.
30. Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, et al. Molecular cloning of human eotaxin, an eosinophilselective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725-30.
31. Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, Langen H, Thelen M, et al. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2171-6.
32. Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137-43.
33. Miyagaki T, Sugaya M, Fujita H, Ohmatsu H, Kakinuma T, Kadono T, et al. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-cell lymphoma. J Invest Dermatol. 2010;130:2304-11.
34. Kleinhans M, Tun-Kyi A, Gilliet M, Kadin ME, Dummer R, Burg G, et al. Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T-cell lymphoma. Blood. 2003;101:1487-93.
35. Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23-33.
36. Picchio MC, Scala E, Pomponi D, Caprini E, Frontani M, Angelucci I, et al. CXCL13 is highly produced by Sèzary cells and enhances their migratory ability via a synergistic mechanism involving CCL19 and CCL21 chemokines. Cancer Res. 2008;68:7137-46.
37. Scala E, Narducci MG, Amerio P, Baliva G, Simoni R, Giovannetti A, et al. T cell receptor-Vbeta analysis identifies a dominant CD60+ CD26- CD49d- T cell clone in the peripheral blood of Sèzary syndrome patients. J Invest Dermatol. 2002;119:193-6.
38. Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, et al. Kinetic investigation of chemokine truncation by CD26/ dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839-45.
39. Narducci MG, Scala E, Bresin A, Caprini E, Picchio MC, Remotti D, et al. Skin homing of Sèzary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107:1108-15.
40. Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. 1999;155:1577-86.
41. Youn BS, Yu KY, Oh J, Lee J, Lee TH, Broxmeyer HE. Role of the CC chemokine receptor 9/TECK interaction in apoptosis. Apoptosis. 2002;7:271-6.
42. Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, et al. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med. 2003;198:1337-47.
43. Kim YH, Hoppe RT. Mycosis fungoides and the Sèzary syndrome. Semin Oncol. 1999;26:276-89..
44. de Coninck EC, Kim YH, Varghese A, Hoppe RT. Clinical characteristics and outcome of patients with extracutaneous mycosis fungoides. J Clin Oncol. 2001;19:779-84.
45. Anadolu RY, Birol A, Sanli H, Erdem C, Tursen U. Mycosis fungoides and Sèzary syndrome: Therapeutic approach and outcome in 113 patients. Int J Dermatol. 2005;44:559-65.
46. Yano H, Ishida T, Inagaki A, Ishii T, Ding J, Kusumoto S, et al. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: A novel immunotherapy for aggressive/refractory mycosis fungoides and Sèzary syndrome. Clin Cancer Res. 2007;13:6494-500.
47. Ishida T, Iida S, Akatsuka Y, Ishii T, Miyazaki M, Komatsu H, et al. The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2004;10:7529-39.
48. Baatar D, Olkhanud P, Newton D, Sumitomo K, Biragyn A. CCR4- expressing T cell tumors can be specifically controlled via delivery of toxins to chemokine receptors. J Immunol. 2007;179:1996-2004.
49. Wu XS, Lonsdorf AS, Hwang ST. Cutaneous T-cell lymphoma: Roles for chemokines and chemokine receptors. J Invest Dermatol. 2009;129:1115-9.
50. Junkins-Hopkins JM. Immunomodulatory therapy of cutaneous Tcell lymphoma: A multimodality approach in advanced disease. J Am Acad Dermatol. 2009;61:1056-8.
Cómo citar
Descargas
Descargas
Publicado
Cómo citar
Número
Sección

| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |






